If Two Covalently Bonded Atoms Are Identical the Bond Is
A shared electron pair. A chemical bond between atoms results from the attraction between electrons and.

Cp Chemistry Mid Term Review What Midterm Grade Do You Expect Be Honest 1 A 2 B 3 C 4 D 5 F Response Grid Ppt Download
But if two atoms have very close electro-negativities then they can give rise to a very nearly non-polar bond.
. C two different ions. If two covalently bonded atoms are identical the bond is. If two covalently bonded atoms are identical the bond is identified as.
If two covalently bonded atoms are identical the bond is identified as Non polar Atoms with a strong attraction for electrons they share with another atoms exhibit. If two covalently bonded atoms are identical the bond is a. The difference in covalent bonding and metallic bonding is that covalent bonds involve the overlap of the valence orbitals between two atoms more if there are delocalized electrons and pi bonding for instance and metallic bonding involves the overlap of the valence orbitals of ALL the metal atoms in the sample.
The strongest covalent bonds between any two identical atoms are to be found in which of the following molecules. If the atoms that share electrons have an unequal attraction for the electrons the bond is called a covalent bond. Ethane gas CH3-CH3 diatomic oxygen gas O2 ethene gas CH2CH2 diatomic hydrogen gas H2 ethyne gas CH2CH2 Start your.
The electrons in a covalent bond are shared equally only when the atoms are identical. If two covalently bonded atoms are identical eg. A covalent bond may also be termed a molecular bond.
Iodine then the bond is a nonpolar covalent bond. What is composed of different kinds of. Books and Authors MCQs.
Hence the bond is non polar. If two covalently bonded atoms are identical the bond is Answer. Ethane gas CH 3-CH 3.
If two covalently bonded atoms are identical the bond is identified asA. A covalent bond consists of. If 2 covalently bonded atoms are identical the bond is identified as nonpolar covalent A covalent bond where there is an unequal attraction for the shared electrons is.
Diatomic oxygen gas O 2. D Lewis structures. If two covalently bonded atoms are identical the bond is identified as _____ _____.
Two iodine atoms See the above figure exhibit equal share of valence electrons equal electron density. Up to 24 cash back ____The chemical bond formed when two atoms share electrons is called an a. If two covalently bonded atoms are identical the bond is a.
Covalent bonds form between two nonmetal atoms with identical or relatively close electronegativity values. If two covalently bonded atoms are identical the bond is identified as. A covalent bond consists of.
If two covalently bonded atoms are identical the bond is identified as _____. Nonpolar covalent a covalent bond in which there is an unequal attraction for the shared electrons is ______. Diatomic nitrogen gas N 2.
Diatomic hydrogen gas H 2. It is true that two atoms of the same element are considered covalently bonded. 2 Nonpolar Covalent bond.
For a covalent bond to be non-polar electron pairs need to be shared approximately equally between two bonded atoms. A a shared electron. In most covalent bonds the electrons are held to a greater extent by one atom than by the other.
A covalent bond in which there is an unequal attraction for the shared electrons isA. D an octet of electrons. If two covalently bonded atoms are identical the bond is identified as.
Leave a Commenton If two covalently bonded atoms are identical the bond is identified as. B a shared electron pair. They can be exactly equivalent as typically happens with identical atoms.
The strongest covalent bonds between any two identical atoms are to be found in which of the following molecules. A chemical bond between atoms results from the attraction between the valence electrons and _____ of different atoms. A covalent bond is which there is an unequal attraction for the shared.
Up to 24 cash back ____ 4. Ethene gas CH 2 CH 2. The atoms must share their electrons equally for the bond to be successful.
If two covalently bonded atoms are identical the bond is nonpolar covalent because they have the same electronegativity How does the table of electronegativity values determine what type of bond will form between two atoms. In General Science Mcqs. - coordinate covalent - polar covalent - dipole covalent - nonpolar covalent.
This type of bond may also be found in other chemical. In which of these compounds is the bond between the atoms NOT a non polar covalent bond. A covalent bond in chemistry is a chemical link between two atoms or ions in which the electron pairs are shared between them.
If two covalently bonded atoms are identical the bond is a. When atoms share electrons the electrical attraction of an atom for the shared electrons is called the atoms a.

The Nature Of Covalent Bonds Chemistry Quizizz
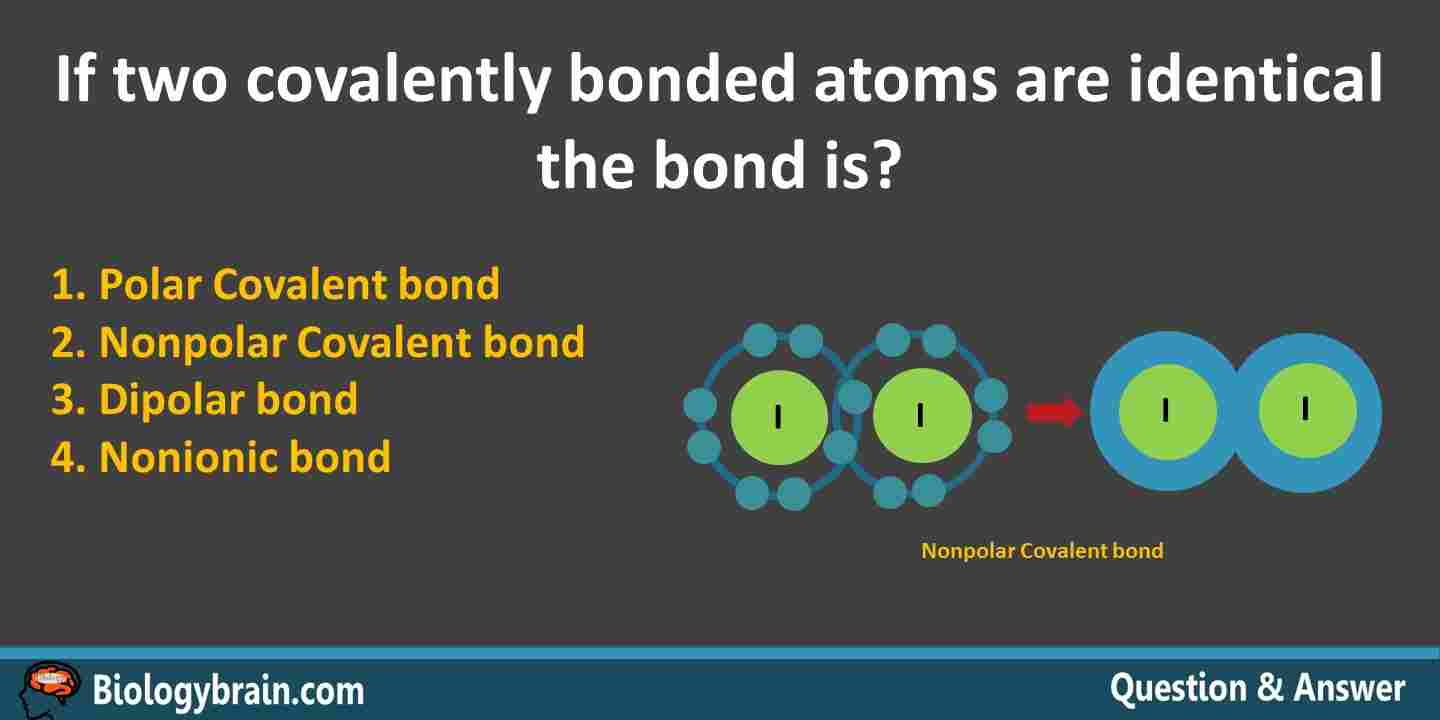
If Two Covalently Bonded Atoms Are Identical The Bond Is Biology Brain

No comments for "If Two Covalently Bonded Atoms Are Identical the Bond Is"
Post a Comment